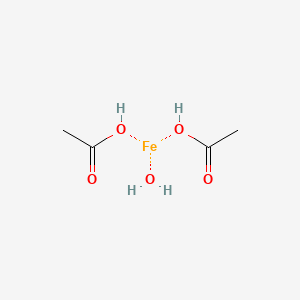
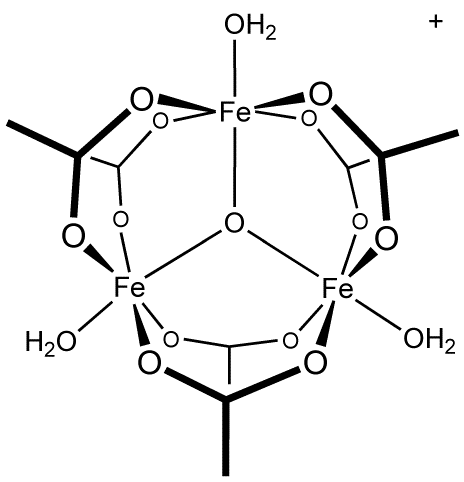
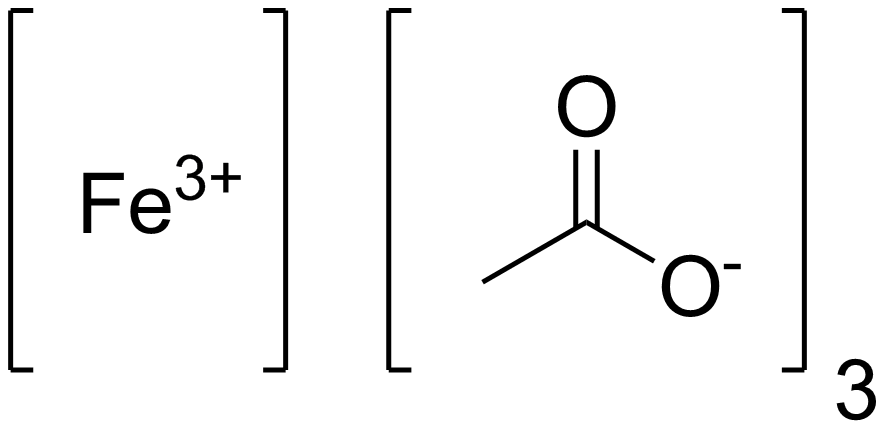
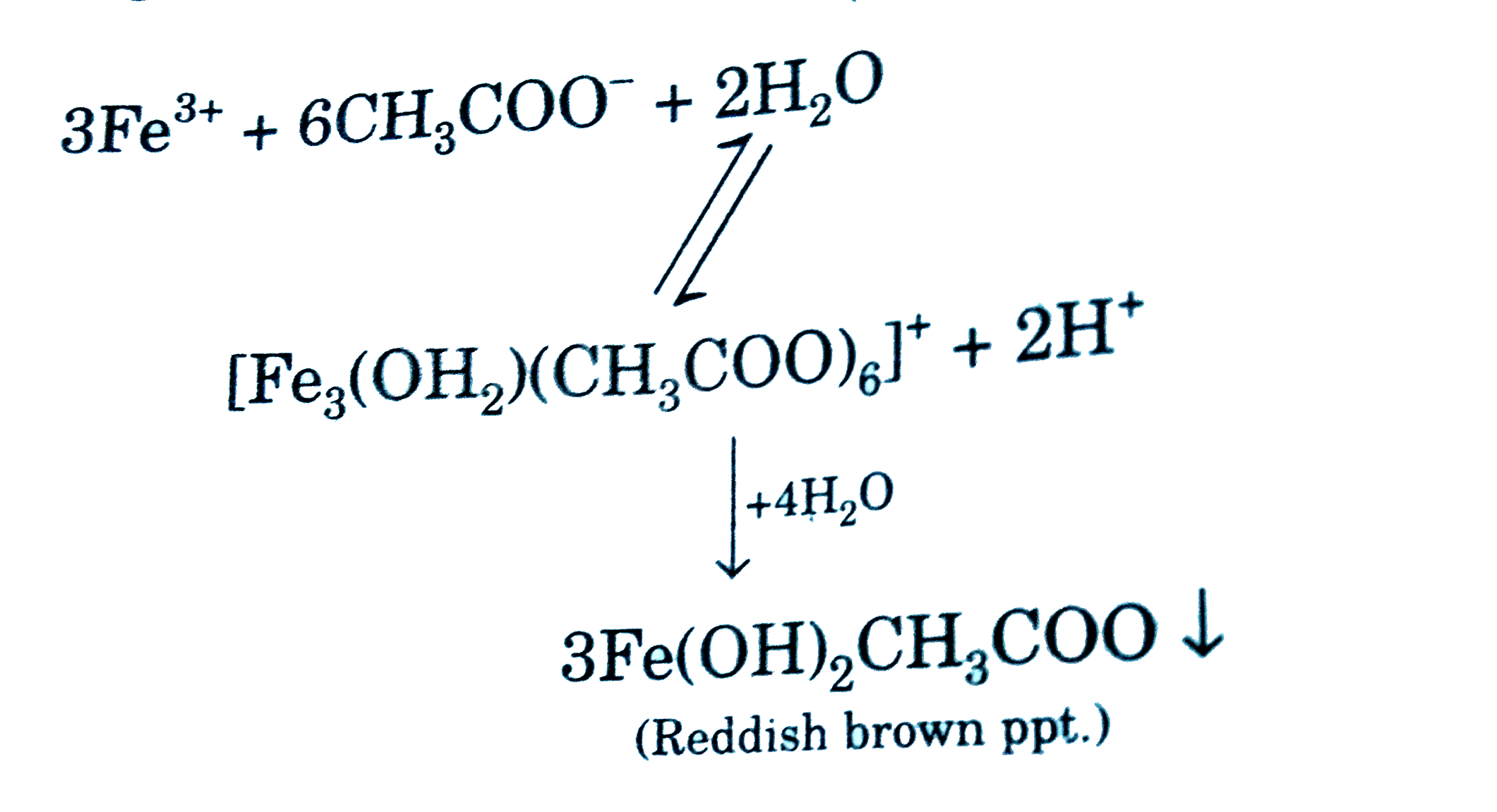Single crystal growth, structural characterization and magnetic properties study of an antiferromagnetic trinuclear iron(III) acetate complex with uncoordinated hexamine - ScienceDirect

Table 1 from Formation peculiarities of iron (III) acetate: potential precursor for iron metal-organic frameworks (MOFs) | Semantic Scholar
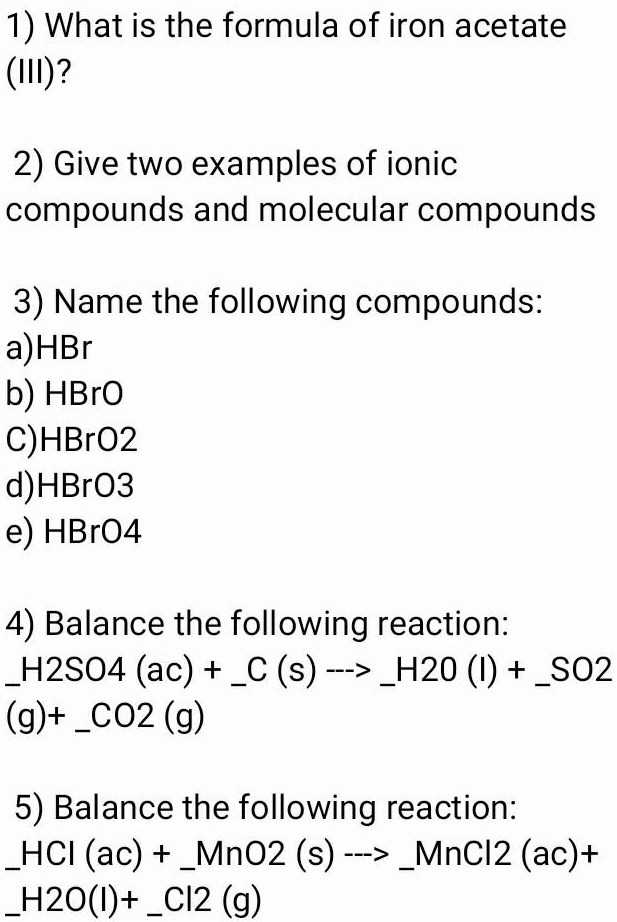
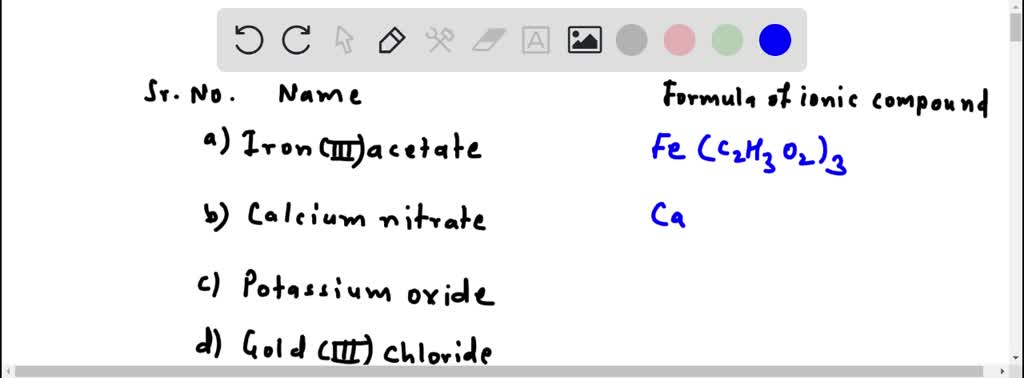
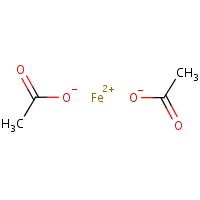
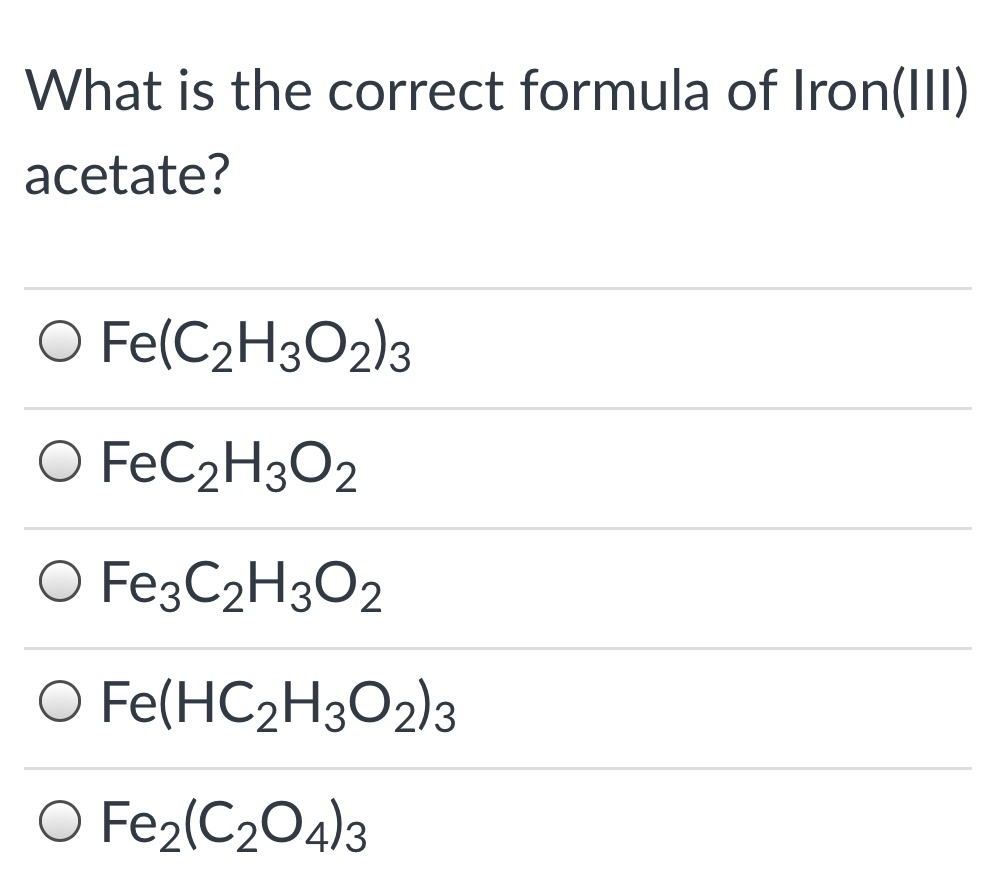
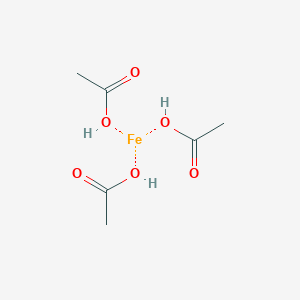
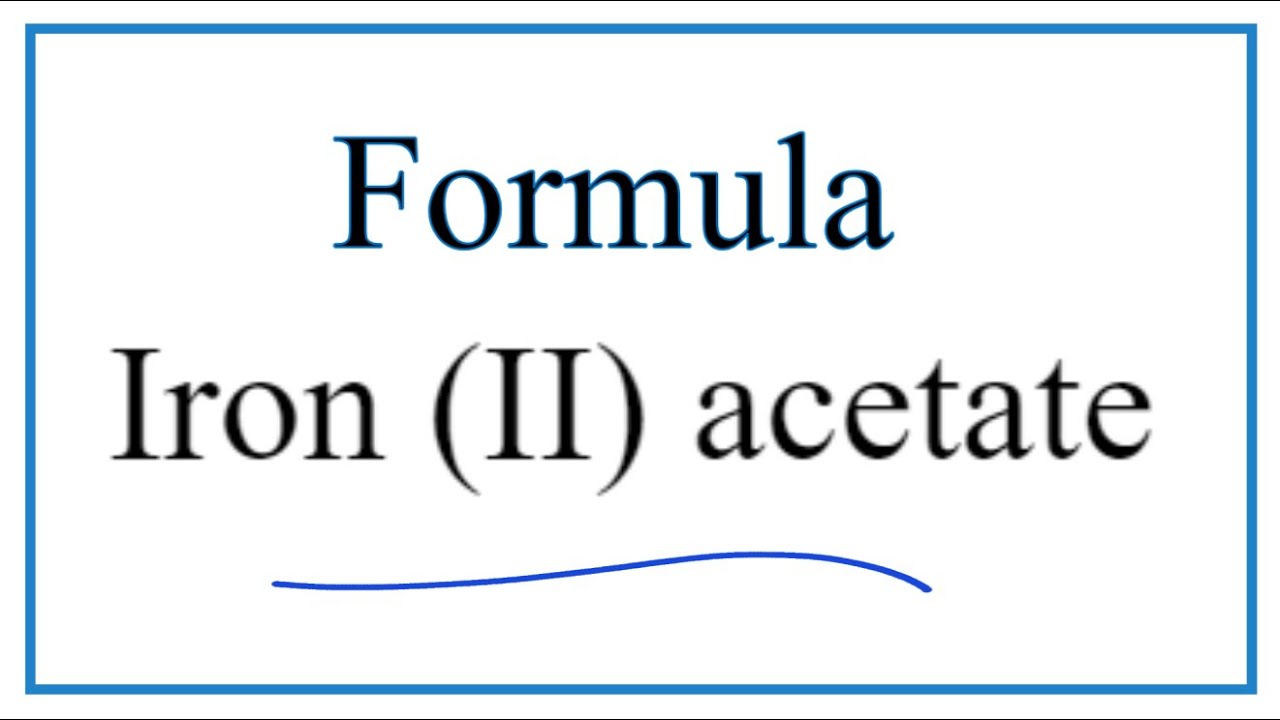
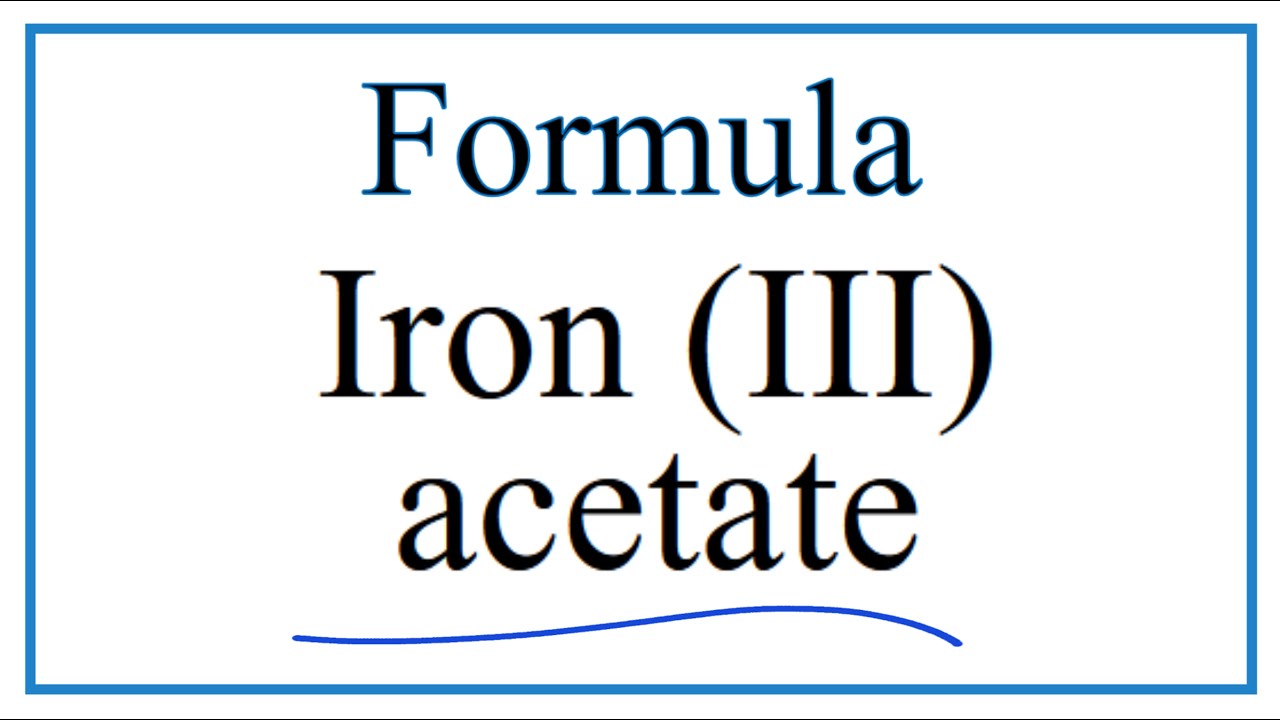
SOLVED: 1) What is the formula of iron acetate (III)? 2) Give two examples of ionic compounds and molecular compounds 3) Name the following compounds: a)HBr b) HBro C)HBroz d)HBro3 e) HBrO4

Compounds vs. Elements Compound Table Salt : Soluble crystals, stable, edible Elements (Components) Sodium – shiny metal, reactive, poisonous Chlorine. - ppt download
![3094-87-9 | Iron(II) Acetate | Ferrous Acetate; Iron acetate [Fe(OAc)2]; Iron Diacetate; Iron(2+) Acetate; Iron(II) Acetate; | C₄H₆O₄Fe | TRC 3094-87-9 | Iron(II) Acetate | Ferrous Acetate; Iron acetate [Fe(OAc)2]; Iron Diacetate; Iron(2+) Acetate; Iron(II) Acetate; | C₄H₆O₄Fe | TRC](https://www.trc-canada.com/prod-img/I775408.png)
3094-87-9 | Iron(II) Acetate | Ferrous Acetate; Iron acetate [Fe(OAc)2]; Iron Diacetate; Iron(2+) Acetate; Iron(II) Acetate; | C₄H₆O₄Fe | TRC
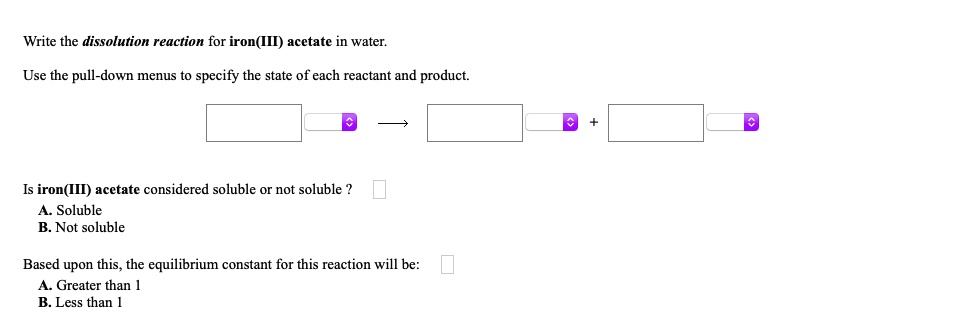
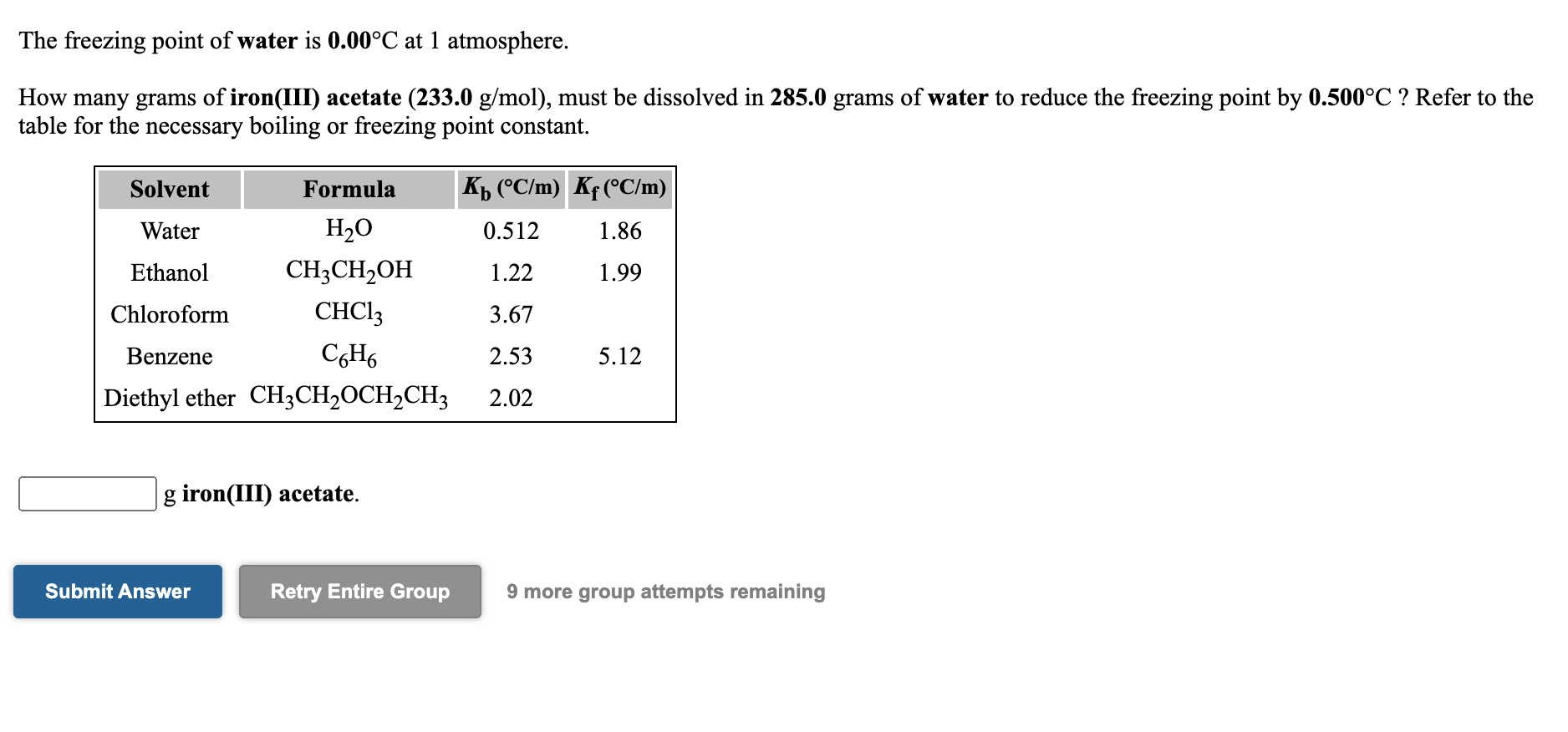
SOLVED: Write the dissolution reaction for iron(III) acetate in water: Use the pull-down menus to specify the state of each reactant and product: Is iron(III) acetate considered soluble or not soluble A.